Objective: The aim of this study was to evaluate the effect of teriparatide (PTH 1-34, rhPTH) on a rabbit defect model with local xenogen grafts histomorphometrically and radiologically.
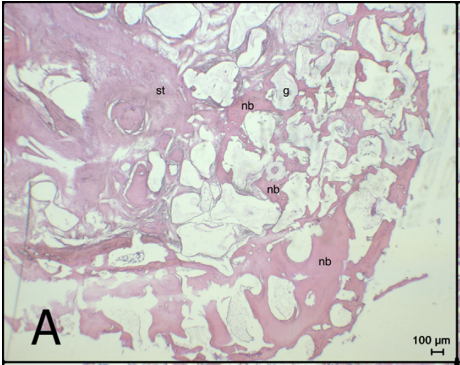
Methods: For this purpose, two 10 mm diameter critical-size defects were created in the calvaria of 16 rabbits. In the control group, the defect area was filled with a xenogen graft, while in the teriparatide group (PTH 1-34), a xenogen graft combination with 20 mcg teriparatide was used. For both 4 e week and 8 e week study groups, new bone, residual graft, and soft tissue areas were evaluated as well as bone volume histomorphometrically and radiologically.
Results: Histomorphometrically, there was a significant difference in new bone area values at the 8th week (p < 0.05), but there was no significant difference between the 4 e week values (p > 0.05). There was no statistically significant difference between the groups at both 4 and 8 weeks (p > 0.05). In the radiologically measured total bone volume values, PTH1-34 group values were found to be significantly higher for both 4 e and 8 e weeks values compared to the control groups (p < 0.05).
Conclusion: In this study, rhPTH, which is used locally in defect areas to be repaired with bone grafts, increases both new bone volume and total bone volume.



.png)
.png)