Objectives: The aim of this study was to describe the cohort of patients who have been treated with Denosumab as neoadjuvant therapy prior to surgery for aggressive giant cell tumor of bone in the extremities, to evaluate the radiological responses to Denosumab comparing Choi criteria and a newly described computerized tomography (CT) classification, and to evaluate the risk of local recurrence after intralesional curettage or radical excision.
Methods: We retrospectively evaluated 36 patients (20 females and 16 males; mean age at diagnosis 36 years (range, 18e64)) treated with neoadjuvant Denosumab therapy prior to surgery for aggressive giant cell tumor of bone in the extremities. The radiological responses to Denosumab treatment were analyzed on the preoperative images after the neoadjuvant course with the Choi criteria and with a newly proposed classification based on CT. All these images were independently reviewed by two of the researchers. Surgical intervention methods were noted and local recurrence rates were evaluated. The correlation between radiological response amount and local recurrence were analyzed for both Choi criteria and the new CT classification.
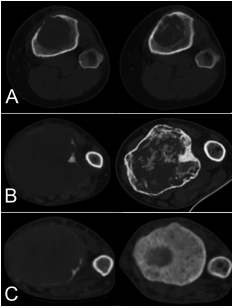
Results: Denosumab was administered for a mean of 21 weeks (range 7e133). Five patients also had a short postoperative course. According to Choi criteria there was a radiological response in 32 patients (89%), while the new CT classification identified responses in all the 36 patients (100%). The identification of changes after 7 weeks of treatment was higher using the CT classification compared to Choi criteria (p ¼ 0.043 vs p ¼ 0.462). The surgical interventions after Denosumab comprised curettage in 29 patients (74%) and resection in 7 (26%). Local recurrence was higher in patients managed with intralesional curettage than in those treated with en bloc resection (55.1% vs 0%, p < 0.001). At last follow up 19 patients (53%) required en bloc resections. Good responders to Denosumab (type 2C) had lower risk of local recurrence (p ¼ 0.047) after either resection or curettage.
Conclusion: The new CT classification evaluated more accurately the response to Denosumab. Our experience suggests that the requirement for radical bone resection remains high despite the use of Denosumab.
Level of evidence: Level IV, Therapeutic Study.



.png)
.png)